How it all started!
In the early 1930s, natural-gas miners started to complain of ice-like material clogging pipelines during transportation of natural gas under cold conditions. It was determined that these crystals were not that of pure ice, but of methane trapped inside "cages" of hydrogen-bonded water molecules!
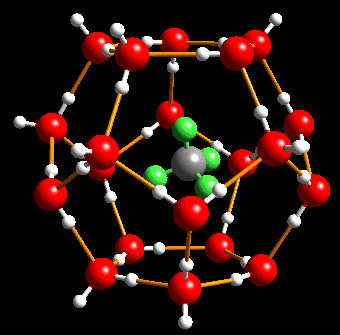
Such gas hydrates or 'gas clathrates' (from Latin word clathratus, bars or lattice) were known for long, being discovered in 1810 by Sir Humphry Davy and were then considered only a laboratery curiosity .
Very soon, ways were found to prevent methane hydrate formation by adding methanol or ethylene glycol to natural-gas pipelines .
But in the 1960s, naturally occurring gas hydrates, methane hydrate or "solid natural gas" were discovered in the Messoyakha gas field in West Siberian Basin. This was significant because naturally occurring gas hydrates were discovered for the first time and what was considerd as scientific curiosity and industrial nuisance, suddenly looked like it might be a significant energy resource.
And what did they find out?
The methane in gas clathrates is primarily generated by bacterial degradation of organic matter in anaerobic environment (anoxic sediments); though, in some regions (e.g., Gulf of Mexico) methane in clathrates may be at least partially derived from thermal degradation of organic matter. Now these clatharates tend to form once methane is saturated in ocean water between coarse sediment grains, and temperature and pressure conditions are right.
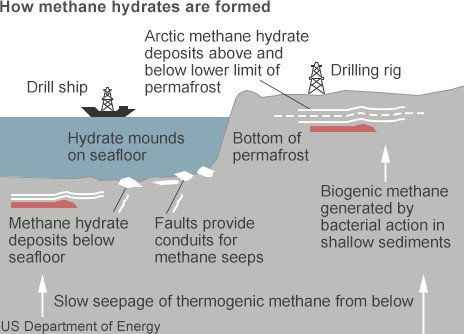
Image source :en.wikipedia.org/wiki/Methane_clathrate
This formation process is determined by temperature and pressure: gas hydrates are stable at low temperatures and/or high pressures. Because of the requirements of pressure and temperature, and because of requirement of relatively large amounts of organic matter for bacterial methanogenesis, clathrates are mainly restricted to two regions: high latitudes (polar regions), and along the continental margins in the oceans (along geological faults allowing for migration of gas from deep)( Kvenvolden, 1998).
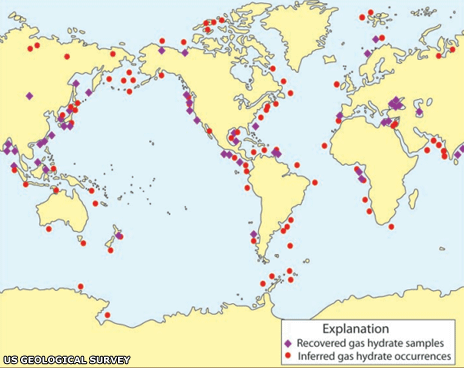
The stakeholders
Many governments (mainly those of the US, Canada, Russia, India and Japan) showed interest in exploring possibilities of hydrates as a fuel.
Oil poor countries like Japan and India started explorations in 1990s for hydrate deposits and ways to utilise these as energy source to achieve the long cherished dream of energy independence.
Initial findings were quite encouraging :
"Estimates suggest that there is about the same amount of carbon in methane hydrates as there is in every other organic carbon store on the planet," says Chris Rochelle of the British Geological Survey.
But, it was the second part of the task: harvesting the resource, which turned out to be Herculean.
The USSR tried unsuccessfully to recover gas hydrates from permafrost reservoirs in the 1960s and 1970s.
It was realized that hydrate extraction is not only technically challenging but, possibly highly risky too.
These hydrates are believed to contribute significantly to the integrity and stability of the geology around a formation. If hydrates be removed, the area around the formation can be significantly destabilised. Risks are especially high where continental margins are concerned as massive landslides can occur if seabed loses its delicately balanced integrity.
Moreover, even if this doesn't happen these hydrates are very unstable and, once removed from high pressure and low temperature environment, tend to decompose very fast (as can be seen in the phase diagram).
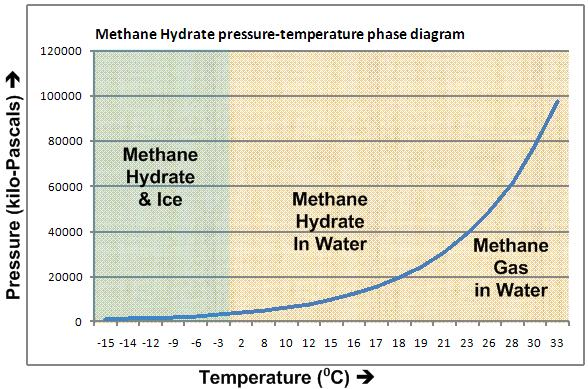
So, methane begins to escape even as it's being transported to the surface. Suitable confining technology is much-needed to prevent resulting pollution.
More recently in 2013, Japan has been successful in extacting methane from extracts by depressurization techniques but this technique cannot be used everywhere else and it is not yet efficient enough.
There is more to the story!
Due to seasonal temperature changes and other minor variations, these hydrates may decompose spontaneously to release small amounts of methane. But with the rising global temperatures, this balance may get tipped. In 2008, about 100 times the normal concentration of releases were reported in the Siberian Arctic.
In 2012, research vessels found about 570 plumes of methane venting from destabilized clathrate stores along the US East Coast . Who is to blame?
The reason has been determined to be warming bottom waters and change in circulation pattern of Gulf Stream – both being results of impacts set off by human-caused climate change.
It is yet to be determined how fast such a release could occur, but the eventual release is likely due to wide-scale clathrate degradation associated with ocean bottom warming. This released methane not only adds upto greenhouse gases in atmosphere but also pollutes seawater, causing anoxia and acidification as methane dissolves in seawater .

(Methane Seep off US East Coast. Image source: Nature.)
As global temperatures rises, warming oceanwater and melting permafrost, such releases will become much more common. In such a case, a catastrophic circular reaction may get triggered as this released methane will contribute to raise temperature further, causing even more methane to escape!
“How long can the gradual warming go on before the methane gets out? Nobody knows, but the longer it goes on, the closer we get to playing Russian roulette,"says Laszlo Varro of the International Energy Agency (IEA).
Methane hydrate: Dirty fuel or energy saviour? By Richard Anderson, Business reporter, BBC News
Mr. Anderson goes on to speculte that 'capturing the methane and burning it suddenly looks like rather a good idea. Maybe this particular hydrocarbon addiction could prove beneficial for us all.'
Interestingly, many think that this may buy us more time as we change modes; from fossil fuels to get into 'sustainable mode', as we develop better energy resoureces technology for clean energy. This can be thought of as an important stepping-stone in our transition to cleaner, greener energy sources.
But there is still more to the story!!!
Even if we are successful to develop technologies required to make this transition, more factors are at play .
“If methane hydrate allows much of the world to switch from oil to gas, the conversion would undermine governments that depend on oil revenues, especially petro-autocracies like Russia, Iran, Venezuela, Iraq, Kuwait, and Saudi Arabia. ............... Worse, most oil nations are so corrupt that social scientists argue over whether there is an inherent bond — a “resource curse” — between big petroleum deposits and political malfeasance. It seems safe to say that few Americans would be upset if a plunge in demand eliminated these countries’ hold over the U.S. economy. But those same people might not relish the global instability — a belt of financial and political turmoil from Venezuela to Turkmenistan — that their collapse could well unleash.”
-What If We Never Run Out of Oil? Charles C. Mann, The Atlantic
Concerns are also raised that trying to develop hydrate related technologies may undermine efforts going on renewable energy resoureces by diverting funds and manpower.
While, these discussions are underway, a bomb is ticking off somewhere. It will be interesting to see if we can afford to burn 'ice' to buy some more time to right our wrongs.
References and for further reading:
-
http://www.theatlantic.com/magazine/archive/2013/05/what-if-we-never-run-out-of-oil/309294/
-
http://science.howstuffworks.com/environmental/green-tech/energy-production/frozen-fuel1.htm
-
http://www.sciencearchive.org.au/nova/newscientist/118ns_003.htm
-
http://www.theatlantic.com/magazine/archive/2013/05/what-if-we-never-run-out-of-oil/309294/

 © Nerd Magazine, IIT Kanpur,2017. All rights reserved.
© Nerd Magazine, IIT Kanpur,2017. All rights reserved.
Comments
comments powered by Disqus