|
Title of Talk Asymmetric Supramolecular-catalysis: Good Protocol to Achieve High Asymmetric Induction from the Functionalized Substrates
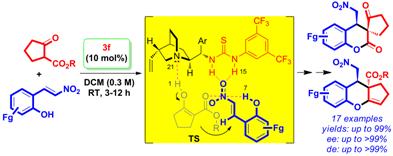
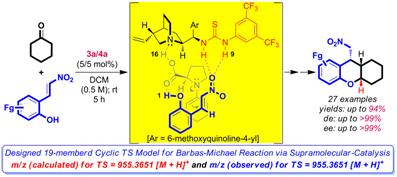
Abstract Utilization of large-size supramolecular rings in the pre-transition state (pre-TS) of enamine- and enol-based Michael reactions for high asymmetric induction will be discussed. Enantiomerically pure, drug-like hexahydroxanthenes and spirocyclic compounds with three contiguous stereocenters were synthesized through supramolecular catalysis by d-proline and quinine-NH-thiourea or quinine-NH-thiourea followed by reductive etherification or lactonization reactions, respectively from functionalized precursors under mild conditions.
Acknowledgments: I sincerely thank all my students for their invaluable contributions to the work described in this talk and I also thank DST, CSIR, UGC and HCU for financial support.
References: 1. D. B. Ramachary, R. Madhavachary and M. S. Prasad, Org. Biomol. Chem., 2012, 10, 5825–5829. 2. D. B. Ramachary, R. Sakthidevi and K. S. Shruthi, Chem. Eur. J., 2012, 18, 8008-8012. |
|
Dhevalapally B. Ramachary, Associate Professor
Catalysis Laboratory, School of Chemistry, University of Hyderabad, Hyderabad-500 046, INDIA e-mail: ramsc@uohyd.ernet.in
His research interests include Synthetic Organic Chemistry (Both Total Synthesis and Reaction Engineering), Organocatalysis (Small molecular catalysis), Development of MCR and MCC Reactions and Application of Organocatalysis in Other Disciplines. He is a recipient of B. M. Birla Science Prize in Chemical Sciences for 2011, Anil Kumar Bose Memorial Award of the INSA 2010, Associate Fellow of the Andhra Pradesh Akademi of Sciences-2010, Member of The National Academy of Sciences, India 2009, and INSA Medal for Young Scientist 2006. |