Our primary research revolves around the 'development of green organometallic reagents and their applications to organic synthesis'. We embarked upon the development of 'new generation cross-coupling reactions' using triarylbismuths as 3-fold coupling reagents. Over the years, we have developed a variety of new coupling reactions using organobismuth chemistry, with a diverse range of reactivity under palladium catalyzed conditions with high atom-economy. Further research activities in our group include convergent organic synthesis, microwave mediated organic synthesis, auto-catalysis, metal catalyzed reactions and other reactions of contemporary interest.
New Generation Green Cross-Coupling Reactions: Cross-coupling methods have enriched the art of organic synthesis and evolved as effective synthetic tools to construct complex molecular systems. The well-known coupling reactions such as Suzuki, Stille, Negeshi, etc. invariably involve only one C-C bond formation (eq. 1).

We envisaged that a paradigm shift in the reagent capability is necessary to make these reactions more green and atom-economic with additional potential for multi C-C couplings in one-pot operation.
Triarylbismuths, which are non-toxic, air stable, and contain three aryl groups (e.g., Figure 1), appeared to us as the most promising green organometallic reagents with 3-fold coupling capabilities (eq.2).
These reagents could, in principle, be employed in sub-stoichiometric 1/3 molar equivalents with increased atom-efficiency in a one-pot operation (eq. 2 vs eq. 1). Our consistent efforts have led to the development of new generation cross-coupling reactions with triarylbismuths reagents, and opened up a plethora of new opportunities in terms of reactivity and selectivity. Thus, a new series of Pd-catalyzed coupling reactions have been developed involving aryl, heteroaryl, acyl, allyl and vinyl couplings, bis-couplings, carbonylations and domino one-pot coupling reactions.
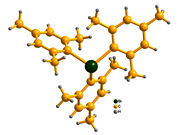
Figure 1.The Structure of trimesityl-bismuthine
 New Office Automation (Pingala)
New Office Automation (Pingala) 








