|
|
|
|
Gurunath Ramanathan
PhD (IISc Bangalore)
|
Email: gurunath[AT]iitk.ac.in
Office Phone: 0512-259-7417
|
Professor, Department of Chemistry
Specialization
Environmental Biochemistry, Peptide chemistry, Biophysics, Protein modelling, bioorganic chemistry, biomedical engineering and Enzymology
Research Interest
- Environmental biodegradation, Biochemistry and microbiology: Our group is isolating microorganisms from industrial and domestic waste water and other environments that specifically degrade certain common chemicals. We have already reported a pseudomonas and an agrobacterium that degrade mono- and di-substituted aromatics. We are now looking at the gene level to study the enzymes that catalyze this metabolism. We have cloned 3-nitrotoluene dioxygenase from diaphorobacter species and are studying the enzyme mechanism.
- Fluorescent Probes in Biology: Our group is interested in discovering new fluorescent probes. Our first generation probes are based on the fluorophore found in the Green Fluorescent Protein (GFP). The imidazolinone fluorophore is formed by the condensation of three amino acids in this protein. We have synthesized several imidazolin-5-ones and are attempting to recover the fluorescence.
- Protein stability and folding: We are also looking at some hyperthermophilic proteins from the thioredoxin family to study their thermal stability.
- Semisynthesis of selenoproteins: We are currently semisynthesizing selenoproteins by peptide- protein ligation. We are synthesizing the peptides containing selenocysteine by solution phase methods and ligating it to the protein produced recombinantly.
- Analysis of protein structure: We are also analyzing the PDB databank for protein motifs. Our analysis is currently then being used to supplement our experimental work.
|
|
|
|

Education
- PhD (1994), IISc, Bangalore.
Thesis Title:Non- protein amino acids in de novo design - an evaluation
Thesis Supervisor:Prof. P. Balaram, Molecular Biophysics Unit, IISc., Bangalore
- M.Sc. (1988), Chemistry, Delhi University
- B.Sc. (Hons.), Chemistry Delhi University

Website(s)

CV
|
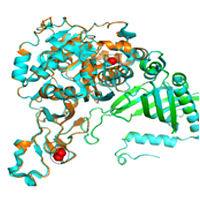
Proteins perform a variety of cellular functions. We study protein function using peptide or organic molecules as models. With our broad interest peptides we are trying to mimic the function of complex proteins through rational design. For this, we use non-protein synthetic amino acids as scaffolds to tailor the peptides to get secondary structure folds to address fundamental aspects of protein folding. Our findings reveal that the interaction of side chains with the main often directs the structure of these molecules both in solution and in solid state. This research led us to the successful use of green fluorescent protein chromophore models in organic solar cells. We are actively involved in isolation of microbes from various environments that specifically target and degrade organic pollutants. In this journey, we recently reported the diaphorobacter species strain DS-2 that degrades 3-nitrotoluene (isolated from an industrial waste treatment plant) and paracoccus strain DMF that completely degrades dimethylformamide (isolated from domestic waste water) and the complete biochemical pathway of degradation of pollutants in these strains was determined. The first degradative enzyme (3-nitrotoluene dixygenase) from diaphorobacater species strain DS-2 which preferentially transforms only 3-nitrotoluene to a mixture 3- and 4- methyl catechols was also cloned by us. This complex protein is a mononuclear iron containing enzyme that has a rieske 2Fe-2S iron-sulfur cluster. It contains two oxygenase subunits (small a -23 kDa and large b-50 kDa), one reductase subunit (35 kDa) and a ferredoxin subunit (12 kDa). The structure of the complete oxygenase subunits using its homology with a known dioxygenase (nitrobenzene dioxygen-ase) has been modeled by us (see figure below). In collaboration, we have recently developed a sensitive detection of microorganisms comprising of an integration of techniques like cell sorting, selective concentration and on-chip real time PCR. Homology modeled oxygenase from diaphorobactersp strain DS-2. The large (a-chain turquoise) and small (b-chain green) of 3-nitrotoluenedioxygenase superposed on the protein nitrobenzene dioxygenase from commomonas sp. JS765. The Rieske iron is circled and the mononuclear iron is shown in the square.
Organic Chemistry, General Chemistry, Communication skills, Biological Chemistry, environmental Chemistry
-
Biomineralization of 3-nitrotoluene by diaphorobacter species, Biodegradation, 24, 645 (2013)
-
Integrated sorting, concentration and real time PCR based detection system for sensitive detection of microorganisms, Sci. Rep. 3, 3266 (2013)
-
Segregation into chiral enantiomeric conformations of an achiral molecule by concomitant polymorphism, Cryst. Growth Des., 12, 1823 (2012)
-
Excited state relaxation dynamics of model green fluorescent protein chromophore analogs: evidence for cis-trans isomerism, J. Phys. Chem. A, 115, 13733 (2011).
-
A change in the 310- to α- helical transition point in the heptapeptides containing sulfur and selenium, Cryst. Growth Des., 11, 2238 (2011)
-
Biomineralization of N,N-Dimethylformamide by paracoccus sp. Strain DMF J. Hazard. Mater., 171, 268-272 (2009)
-
A patent on an improved organic optoelectronic device has been granted at Delhi, Ref. No., 3263/RQ – DEL 2007
-
Professor, IIT Kanpur, 2011-.
-
Associate Professor, IIT Kanpur, 2008-2011
-
Assistant Professor, IIT Kanpur 2000-2007
-
Sveriges Lantbruks Universitet, Uppsala, Sweden 1999-2000
-
Karolinska Institutet, Stockholm, Sweden, 1996-1999
-
Post docs at MIT, Cambridge, USA, 1995-1996;
-
Life Member CRSI
-
Life Member Indian Peptide society
-
Life member NMR society of India Member, American Chemical Society
Office
CL 203E ,
Department of Chemistry
IIT Kanpur,
Kanpur 208016
Office Phone: 0512-259-7417 (O)
Email: gurunath[AT]iitk.ac.in
|
|
|












